Potential underutilisation of PTE in Singapore
07 Aug 2024
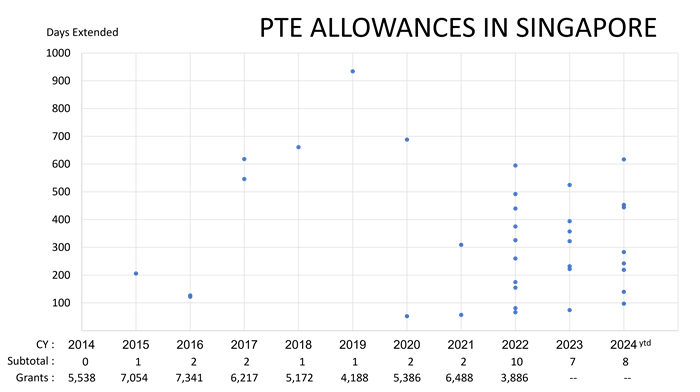
(Chart data is from Patents Journals and IP Statistics available on the IPOS website as of mid-July 2024.)
Since 2014, there have been only 36 Patent Term Extension (PTE) allowances for over 51,000 patents granted by the Intellectual Property Office of Singapore (IPOS) during that same decade. In calendar year 2022, the year with the largest number of PTE allowances, PTE was allowed in less than a quarter of a percent of the granted patents for that year. The average number of days extended for these 36 patents was less than one year (at 331 days), and the mean number of days extended was less than eight months (at 242 days).
The legal basis for the PTE allowances can be confirmed in 24 of these 36 patents based on “Patent Open Dosser” documents accessible from the IPOS website. These online records document only PTE allowances based on unreasonable delay in the prosecution of patent applications. There are no online records documenting a PTE allowance based on unreasonable curtailment of patent exploitation based on delay in the processing by Health Sciences Authority (HSA) of an application for marketing approval for a patented pharmaceutical product.
The low number of PTE allowances is a testament to diligent processing of patent applications by IPOS and marketing approval applications by HSA. From my own experience, IPOS decisions on eligibility for grant can be as short as 22 days from first filing (see, for example, SG Patent No. 10201908409R), and HSA marketing approval cycle time is typically in the range of 14 to 18 months.
The 4x uptick in the annual PTE allowance rate starting 2022 is attributable to the efforts of a single law firm in Singapore. This single law firm is listed as the registered patent agent in 20 of the 24 patents receiving PTE allowances between 2022 and mid-2024. Such data is an indication that PTE allowance is potentially underutilised within Singapore.
PTE is available in Singapore based on unreasonable delay in the prosecution of a patent application or, alternatively, based on unreasonable curtailment of patent exploitation attributable to HSA based on delay in the processing of an application for marketing approval for a patented pharmaceutical product. Pursuant to the Singapore patent provisions, minimal requirements for PTE mandate one of the following:
-
Supplementary Examination. Where the application undergoes Supplementary Examination by IPOS, PTE is not available unless: (a) the interval between the date of filing and the grant date of patent (excluding periods attributable to the applicant) exceeded four years; or (b) the patent office that granted the corresponding patent or related national phase patent extended that foreign patent.
-
Local Examination. Where the application undergoes local examination by IPOS, PTE is not available unless: (a) the interval between the date of filing and the grant date of patent (excluding periods attributable to the applicant) exceeded four years; or (b) the interval between the date of request for examination and the grant date of patent (excluding periods attributable to the applicant) exceeded two years.
-
HSA Curtailment. Where HSA has processed an application for marketing approval for a patented pharmaceutical product, PTE is not available unless the HSA marketing approval application processing time (excluding periods attributable to the applicant) exceeded two years.
Unlike the automatic processing of “Patent Term Adjustment” by the U.S. PTO, the Singapore provisions require unilateral action by the patent proprietor. PTE must be requested by the patent proprietor from IPOS within 6 months from the date of the grant of the patent by IPOS or within 6 months from the date marketing approval was obtained from HSA. Hence, patent proprietors interested in PTE should seek prompt legal advice from their Singapore legal counsel regarding potential PTE eligibility after recent patent grants and after recent HSA marketing approvals.
The content in this posting is for general informational purposes only. This posting is not intended to constitute or to be relied upon as legal advice. You should consult Singapore legal counsel if you require legal advice regarding Singapore patent practice. The information and opinions expressed in this posting are those of myself and do not necessarily represent the view of my employer Drew & Napier LLC.
- Written by Patrick W. Duncan, Senior Patent Associate at Drew & Napier LLC